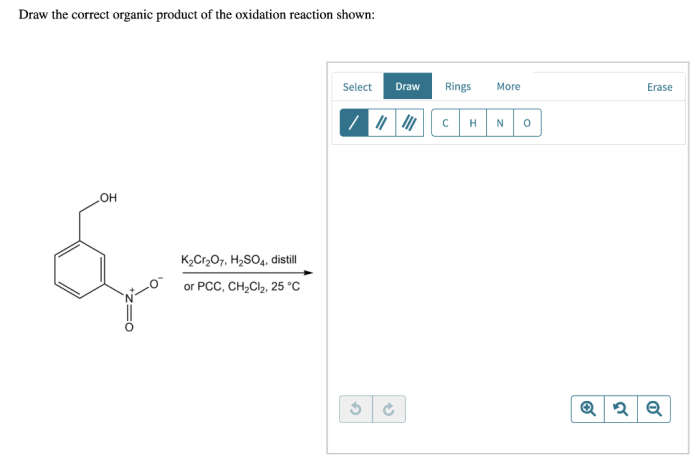
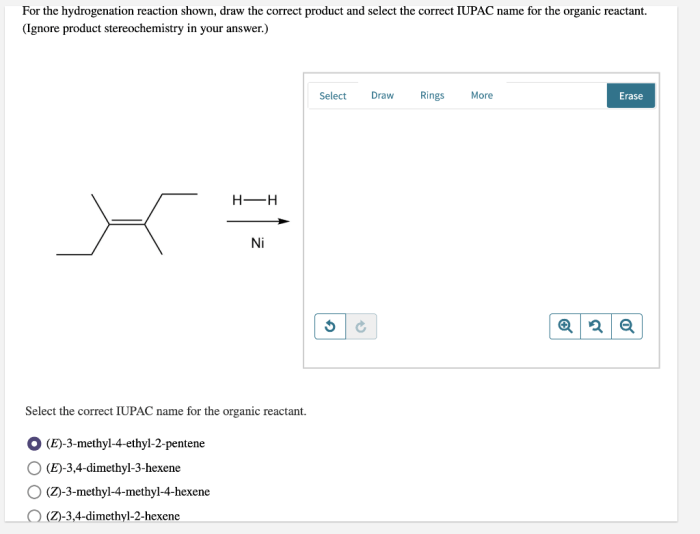
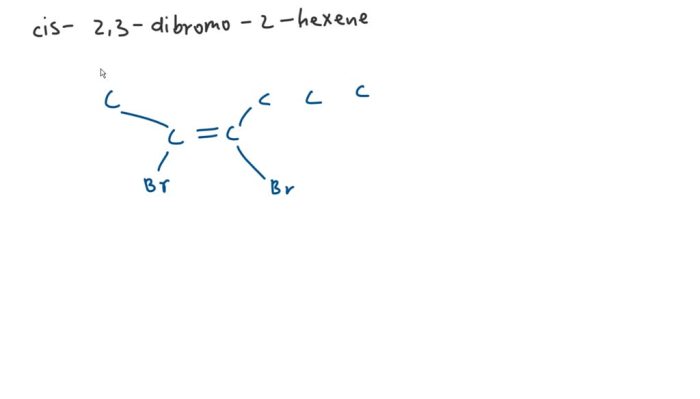
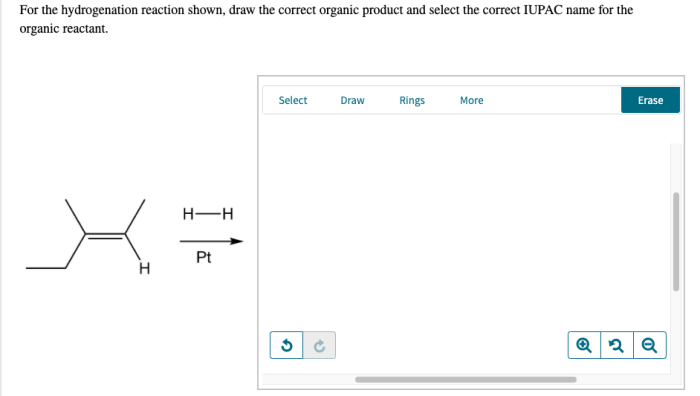
In the realm of organic chemistry, the ability to accurately predict the outcome of reactions is paramount. Draw the correct organic product for the reaction shown. This article delves into the intricacies of reaction analysis, providing a comprehensive guide to identifying the correct organic product.
Understanding the principles of organic product identification is crucial for comprehending the behavior of chemical reactions and their applications in various fields, including pharmaceuticals, materials science, and biotechnology.
Organic Product Identification
In chemical reactions, the organic product is the compound that is formed as the primary result of the reaction. Organic products are typically characterized by their carbon-containing molecular structure and their covalent bonding.
Examples of organic products include:
- Methane (CH4)
- Ethanol (C2H5OH)
- Benzene (C6H6)
Identifying the correct organic product is essential for understanding the outcome of a reaction and predicting the properties of the resulting compound.
Reaction Analysis
Analyzing chemical reactions is crucial for predicting the products that will be formed. By understanding the reagents, catalysts, and reaction conditions, it is possible to determine the most likely outcome of a reaction.
Methods for analyzing reactions include:
- Using reaction mechanisms
- Drawing energy diagrams
- Considering the electronic structure of the reactants
Drawing Organic Products
Drawing organic structures is essential for communicating chemical information. There are several conventions for drawing organic structures, including:
- Structural formulas
- Condensed formulas
- Line-angle formulas
Each of these conventions has its own advantages and disadvantages, and the choice of which convention to use depends on the specific purpose of the drawing.
Reaction Mechanisms
Reaction mechanisms are detailed descriptions of the steps that occur during a chemical reaction. They provide insight into the electronic and structural changes that take place as the reactants are converted into products.
Different types of reaction mechanisms include:
- Nucleophilic substitution
- Electrophilic addition
- Elimination reactions
Understanding reaction mechanisms is essential for predicting the products of a reaction and for designing new synthetic methods.
Regioselectivity and Stereoselectivity, Draw the correct organic product for the reaction shown.
Regioselectivity and stereoselectivity are two important concepts in organic chemistry that describe the selectivity of a reaction for a particular regioisomer or stereoisomer.
Factors that influence regioselectivity and stereoselectivity include:
- The electronic structure of the reactants
- The reaction conditions
- The presence of catalysts
Understanding regioselectivity and stereoselectivity is essential for controlling the outcome of organic reactions and for synthesizing specific compounds with desired properties.
Quick FAQs: Draw The Correct Organic Product For The Reaction Shown.
What is the significance of identifying the correct organic product?
Identifying the correct organic product is essential for understanding the outcome of reactions and designing synthetic strategies. It allows chemists to predict the properties and behavior of the product, which is crucial for applications in various fields.
How can reaction mechanisms help in predicting organic products?
Reaction mechanisms provide a detailed understanding of the steps involved in a reaction, including the formation and breaking of bonds. By analyzing reaction mechanisms, chemists can identify the intermediates and transition states, which allows them to predict the most likely outcome of the reaction.
What factors influence regioselectivity and stereoselectivity in organic reactions?
Regioselectivity and stereoselectivity are influenced by various factors, including the electronic properties of the reactants, the reaction conditions, and the presence of catalysts. Understanding these factors enables chemists to control the regio- and stereochemical outcome of reactions and obtain the desired products.