Embark on an enlightening journey through the realm of chemistry with our comprehensive average atomic mass calculations worksheet. Delve into the intricacies of atomic mass and its profound significance, unraveling the secrets behind the weighted average approach that lies at the heart of these calculations.
Prepare to master the step-by-step process of determining average atomic mass, gaining invaluable insights into its diverse applications and the nuances that shape its accuracy.
Throughout this worksheet, you will encounter real-world examples that illuminate the practical significance of average atomic mass, solidifying your understanding of its role in molecular weight determinations, stoichiometry, and a myriad of other chemical calculations. By the end of this exploration, you will emerge as a confident and proficient chemist, equipped with the knowledge and skills to navigate the complexities of atomic mass calculations with precision and ease.
Understanding Atomic Mass Calculations
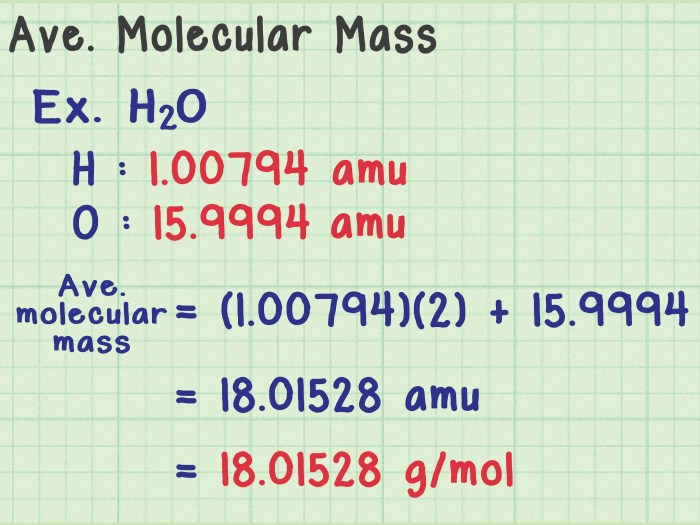
Atomic mass, a fundamental concept in chemistry, represents the average mass of all the atoms of an element, taking into account the different isotopes and their relative abundances. This concept is crucial for determining the mass of molecules and predicting chemical behavior.
The weighted average, a statistical method, is employed to calculate atomic mass. Each isotope’s mass is multiplied by its abundance, and the results are summed. The sum is then divided by the total number of atoms to obtain the average atomic mass.
Steps for Calculating Average Atomic Mass
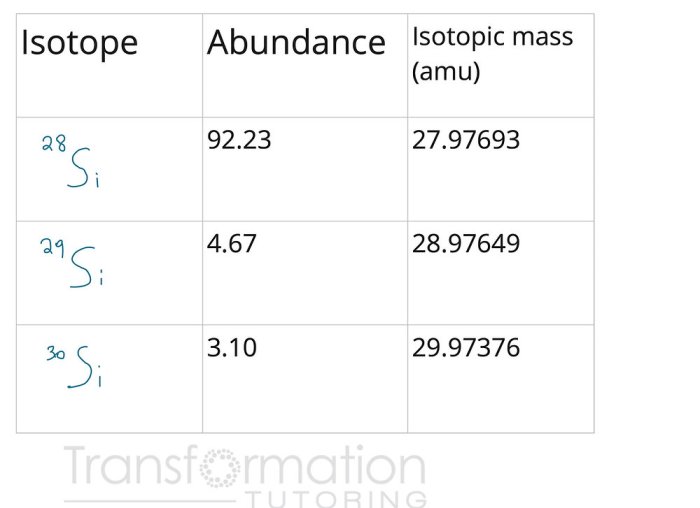
- Identify the isotopes of the element and their respective atomic masses.
- Determine the abundance of each isotope, typically expressed as a percentage or decimal fraction.
- Multiply the atomic mass of each isotope by its abundance.
- Sum the products obtained in step 3.
- Divide the sum by the total number of atoms to obtain the average atomic mass.
Example Calculations
| Isotope | Abundance | Atomic Mass (amu) | Product (amu) |
|---|---|---|---|
| 12C | 98.93% | 12.00000 | 11.87058 |
| 13C | 1.07% | 13.00335 | 0.13945 |
| Total | 12.01003 | ||
The average atomic mass of carbon is 12.01003 amu.
Applications of Average Atomic Mass
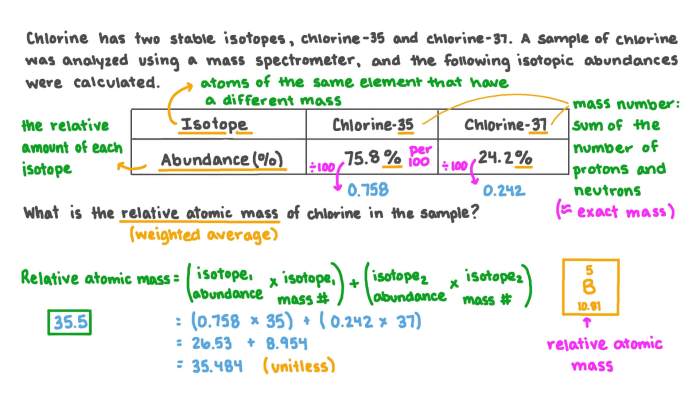
- Determining the molecular weight of compounds.
- Calculating stoichiometric ratios in chemical reactions.
- Predicting the density and other physical properties of substances.
- The average atomic mass is an approximation, as it does not account for isotopic variations within a sample.
- The accuracy of the calculation depends on the availability of accurate isotopic abundance data.
Limitations and Considerations: Average Atomic Mass Calculations Worksheet
Helpful Answers
What is the significance of average atomic mass in chemistry?
Average atomic mass plays a pivotal role in chemistry, providing a representative value for the mass of an element’s atoms, taking into account the contributions of its various isotopes and their relative abundances. This value is essential for accurate calculations involving molecular weights, stoichiometry, and a wide range of other chemical applications.
Can you explain the concept of weighted average in the context of atomic mass calculations?
In atomic mass calculations, the weighted average approach considers both the mass and abundance of each isotope of an element. Each isotope’s mass is multiplied by its abundance, and the resulting values are summed. This sum is then divided by the total abundance of all isotopes to obtain the average atomic mass, which represents the average mass of the element’s atoms.
What are some limitations and considerations associated with using average atomic mass?
While average atomic mass provides a useful approximation, it is important to note that it may not always accurately reflect the actual mass of an individual atom. Factors such as isotopic fractionation and the presence of radioactive isotopes can influence the accuracy of the average atomic mass.
Additionally, the average atomic mass of an element can vary slightly depending on the source of the sample.
