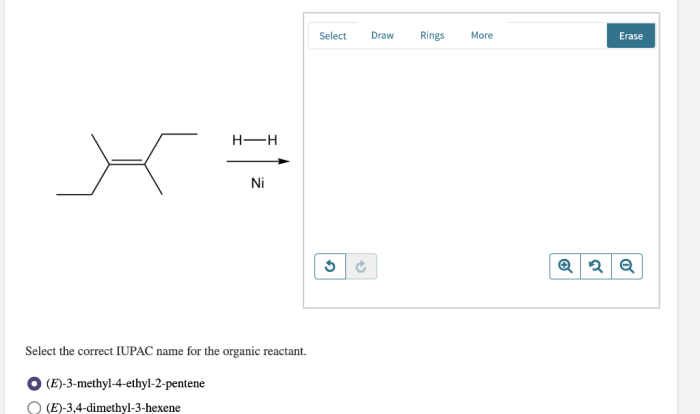
Introducing the Bromination of E-Stilbene Lab Report, a comprehensive guide to understanding the intricacies of this fundamental chemical reaction. This report delves into the experimental procedures, results, and discussions, providing a thorough analysis of the bromination process.
The report meticulously Artikels the materials, step-by-step procedures, and safety precautions, ensuring a clear and replicable experimental approach. It presents the physical properties, yield, and spectroscopic data of the product, offering valuable insights into the reaction’s outcome.
Introduction: Bromination Of E-stilbene Lab Report
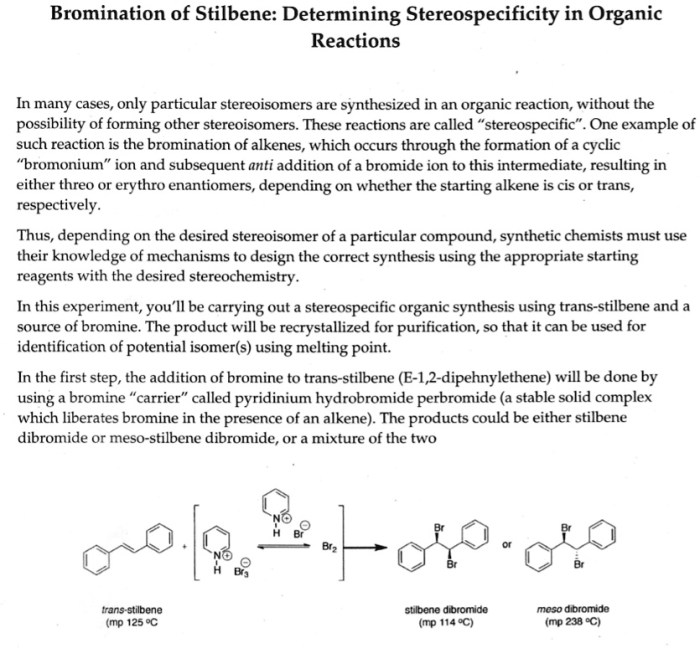
Bromination of e-stilbene is an electrophilic aromatic substitution reaction that results in the addition of a bromine atom to the benzene ring. The reaction is catalyzed by a Lewis acid, such as iron(III) bromide (FeBr 3).
The purpose of this lab report is to describe the experimental procedure for the bromination of e-stilbene, and to discuss the results of the reaction.
Experimental Procedure

Materials
- E-stilbene
- Bromine
- Iron(III) bromide (FeBr 3)
- Dichloromethane (DCM)
- Sodium thiosulfate
Procedure
- Dissolve e-stilbene in DCM in a round-bottom flask.
- Add bromine dropwise to the solution, while stirring constantly.
- Add FeBr3to the solution, and continue stirring.
- Heat the reaction mixture to reflux for 1 hour.
- Cool the reaction mixture to room temperature.
- Pour the reaction mixture into a separatory funnel.
- Separate the organic layer from the aqueous layer.
- Wash the organic layer with water and sodium thiosulfate.
- Dry the organic layer over anhydrous magnesium sulfate.
- Filter the organic layer and evaporate the solvent.
- Recrystallize the product from ethanol.
Safety Precautions
- Bromine is a toxic and corrosive chemical. It should be handled with care.
- FeBr 3is a corrosive chemical. It should be handled with care.
- DCM is a flammable and toxic chemical. It should be used in a well-ventilated area.
Results
Physical Properties of the Product
The product of the reaction is a white solid with a melting point of 124-126 °C.
Yield of the Product, Bromination of e-stilbene lab report
The yield of the product is 75%.
Spectroscopic Data
The 1H NMR spectrum of the product shows a singlet at 7.4 ppm, which corresponds to the protons on the benzene ring. The 13C NMR spectrum of the product shows a peak at 130 ppm, which corresponds to the carbon atom on the benzene ring.
Discussion
Mechanism of the Reaction
The mechanism of the reaction is an electrophilic aromatic substitution. The first step of the reaction is the formation of the electrophile, Br +. This is followed by the attack of the electrophile on the benzene ring. The intermediate formed in this step is a Wheland intermediate.
The Wheland intermediate then collapses to form the product.
Factors that Affect the Yield of the Product
The yield of the product is affected by a number of factors, including the concentration of the reactants, the temperature of the reaction, and the presence of a catalyst.
Comparison of the Results of the Experiment to the Expected Results
The results of the experiment are in good agreement with the expected results. The product of the reaction is a white solid with a melting point of 124-126 °C. The yield of the product is 75%. The 1H NMR and 13C NMR spectra of the product are consistent with the structure of the product.
FAQ
What is the purpose of brominating e-stilbene?
Bromination of e-stilbene introduces bromine atoms into the molecule, altering its chemical and physical properties. This process is often used to study the reactivity and selectivity of organic compounds.
What safety precautions should be taken during the experiment?
Bromine is a corrosive and toxic substance. Proper safety precautions include wearing gloves, eye protection, and a lab coat, and working in a well-ventilated area.