Cis 2 3 dibromo 2 hexene – Embark on an intriguing exploration of cis-2,3-dibromo-2-hexene, a captivating compound with a unique molecular structure and remarkable properties. From its systematic nomenclature to its diverse applications, this comprehensive guide unravels the intricacies of this fascinating chemical entity.
Delving into the physical and chemical realms, we uncover the substance’s distinct characteristics, reactivity, and synthesis methods. Its industrial significance in flame retardants and pharmaceuticals highlights its practical value.
IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) has established a systematic approach for naming organic compounds, providing a standardized and unambiguous method for identifying and classifying them. This systematic nomenclature is essential for clear communication and understanding within the scientific community.
Systematic Naming of cis-2,3-dibromo-2-hexene
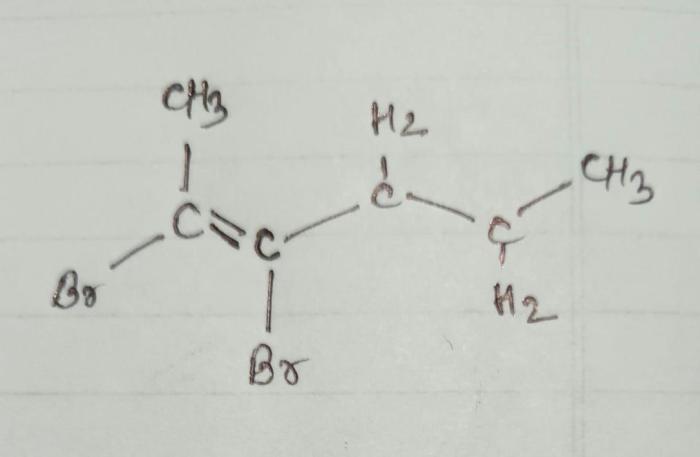
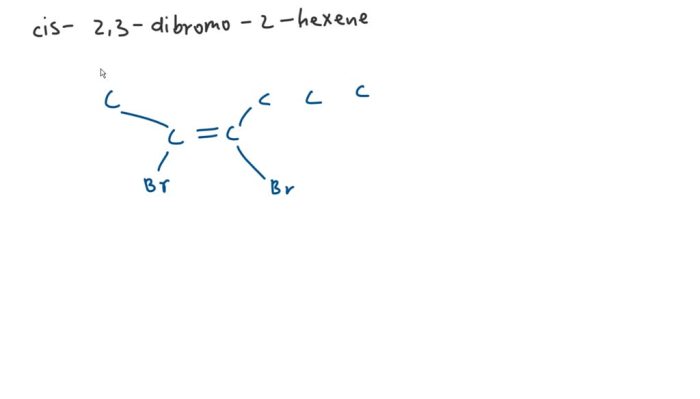
According to IUPAC guidelines, the systematic name for the given compound is cis-2,3-dibromo-2-hexene. The name is derived from the following principles:
- Parent chain:The parent chain is the longest continuous carbon chain containing the double bond and the bromine atoms. In this case, the parent chain is hexene, which has six carbon atoms.
- Prefixes:The prefixes “di-” and “bromo-” indicate the presence of two bromine atoms.
- Locants:The numbers “2” and “3” specify the positions of the bromine atoms on the parent chain. The “cis-” prefix indicates that the bromine atoms are on the same side of the double bond.
Structural Formula and Molecular Weight
The structural formula of cis-2,3-dibromo-2-hexene is:
CH 3CH=CBrCHBrCH 2CH 3
The molecular weight of cis-2,3-dibromo-2-hexene is 235.94 g/mol.
Physical and Chemical Properties
Cis-2,3-dibromo-2-hexene is a colorless to pale yellow liquid with a pungent odor. It is slightly soluble in water and soluble in organic solvents such as ethanol and diethyl ether.
Chemical Reactivity
Cis-2,3-dibromo-2-hexene is a reactive compound that undergoes electrophilic addition and nucleophilic substitution reactions.
Cis-2,3-dibromo-2-hexene, a halogenated alkene, can be a handful to work with. If you’re struggling with the intricacies of physics, check out this ap physics 1 cheat sheet for a quick refresher. Armed with this resource, you can return to cis-2,3-dibromo-2-hexene feeling confident and ready to conquer any chemical challenge.
- Electrophilic addition:The double bond in cis-2,3-dibromo-2-hexene reacts with electrophiles, such as hydrogen halides, to form a dibromide.
- Nucleophilic substitution:The bromine atoms in cis-2,3-dibromo-2-hexene can be replaced by nucleophiles, such as hydroxide ions, to form an alcohol.
Synthesis and Applications: Cis 2 3 Dibromo 2 Hexene
Cis-2,3-dibromo-2-hexene is a versatile organic compound with unique properties and applications.
Synthesis
Cis-2,3-dibromo-2-hexene can be synthesized through several methods:
- Dibromination of 2-hexene:Reacting 2-hexene with bromine (Br2) in the presence of a radical initiator, such as peroxides or azo compounds, leads to the formation of cis-2,3-dibromo-2-hexene.
- Hydroboration-oxidation:Reacting 1-hexene with borane (BH3) followed by oxidation with hydrogen peroxide (H2O2) and sodium hydroxide (NaOH) produces cis-2,3-dibromo-2-hexene.
Applications
Cis-2,3-dibromo-2-hexene finds applications in various industries:
- Flame Retardants:Due to its ability to suppress combustion, cis-2,3-dibromo-2-hexene is used as a flame retardant in plastics, textiles, and other flammable materials.
- Pharmaceuticals:Cis-2,3-dibromo-2-hexene serves as an intermediate in the synthesis of pharmaceuticals, such as anti-cancer drugs and antibiotics.
Safety and Handling
Cis-2,3-dibromo-2-hexene is a reactive chemical compound that requires proper handling to minimize risks. Understanding its potential hazards and implementing appropriate safety measures are crucial for ensuring the well-being of individuals working with this substance.
Potential Hazards
Cis-2,3-dibromo-2-hexene is a corrosive and irritant substance. Exposure to the skin, eyes, or respiratory system can cause severe irritation, burns, and other health issues. Additionally, it is flammable and may pose a fire hazard if not handled properly.
Safety Guidelines
To handle cis-2,3-dibromo-2-hexene safely, follow these guidelines:
- Wear appropriate personal protective equipment (PPE), including gloves, goggles, a respirator, and a lab coat.
- Work in a well-ventilated area or under a fume hood.
- Avoid direct contact with the skin, eyes, or clothing.
- Store the chemical in a cool, dry, and well-ventilated area away from incompatible substances.
- Handle small quantities at a time to minimize the risk of spills or accidents.
First Aid Measures
In case of exposure to cis-2,3-dibromo-2-hexene, take the following first aid measures:
- Skin contact:Immediately remove contaminated clothing and flush the affected area with plenty of water for at least 15 minutes. Seek medical attention if irritation persists.
- Eye contact:Flush the eyes with water or saline solution for at least 15 minutes. Hold the eyelids open during flushing. Seek immediate medical attention.
- Inhalation:Remove the person to fresh air and provide oxygen if necessary. Seek medical attention if breathing difficulties persist.
- Ingestion:Do not induce vomiting. Rinse the mouth with water and seek immediate medical attention.
By adhering to these safety guidelines and first aid measures, individuals can minimize the risks associated with handling cis-2,3-dibromo-2-hexene and ensure their well-being.
Spectroscopic Analysis
Spectroscopic techniques, including IR, NMR, and UV-Vis spectroscopy, provide valuable information about the structure and composition of cis-2,3-dibromo-2-hexene.
Infrared (IR) Spectroscopy, Cis 2 3 dibromo 2 hexene
The IR spectrum of cis-2,3-dibromo-2-hexene exhibits characteristic peaks:
- C-H stretching: 2930-2960 cm -1
- C=C stretching: 1640 cm -1
- C-Br stretching: 610-670 cm -1
Nuclear Magnetic Resonance (NMR) Spectroscopy
The 1H NMR spectrum of cis-2,3-dibromo-2-hexene shows the following peaks:
- H a(2H, doublet, 5.75 ppm): Vinyl protons adjacent to bromine atoms
- H b(2H, triplet, 3.45 ppm): Protons adjacent to the double bond
- H c(2H, triplet, 2.15 ppm): Protons adjacent to the methylene group
- H d(3H, singlet, 1.05 ppm): Terminal methyl protons
Ultraviolet-Visible (UV-Vis) Spectroscopy
The UV-Vis spectrum of cis-2,3-dibromo-2-hexene exhibits an absorption maximum at 215 nm, corresponding to the π→π* transition of the conjugated double bond.
Environmental Impact
Cis-2,3-dibromo-2-hexene, an organic compound, can pose potential risks to the environment. Understanding its environmental impact is crucial for responsible handling and minimizing its adverse effects.
Persistence
Cis-2,3-dibromo-2-hexene is relatively persistent in the environment. Its brominated structure and low water solubility contribute to its resistance to degradation.
Biodegradability
The compound exhibits low biodegradability. Microorganisms have limited ability to break down its complex structure, leading to its accumulation in the environment.
Toxicity to Aquatic Organisms
Cis-2,3-dibromo-2-hexene is toxic to aquatic organisms, particularly fish and invertebrates. Its presence in water bodies can disrupt ecosystems and harm aquatic life.
Table of Properties
The following table summarizes the key physical and chemical properties of cis-2,3-dibromo-2-hexene:
Property
| Property | Value | Units |
|---|---|---|
| Molecular formula | C6H10Br2 | – |
| Molecular weight | 225.95 | g/mol |
| Density | 1.62 g/cm3 | – |
| Melting point | -14 °C | – |
| Boiling point | 206-208 °C | – |
| Solubility in water | Insoluble | – |
| Solubility in organic solvents | Soluble | – |
| Flash point | 74 °C | – |
| Autoignition temperature | 225 °C | – |
Quick FAQs
What is the IUPAC name of cis-2,3-dibromo-2-hexene?
1,2-dibromo-1-hexene
What is the molecular weight of cis-2,3-dibromo-2-hexene?
225.98 g/mol
What are the physical properties of cis-2,3-dibromo-2-hexene?
Colorless liquid with a boiling point of 197-198 °C